KID REPORTERS’ NOTEBOOK
Two New Vaccines Offer Hope
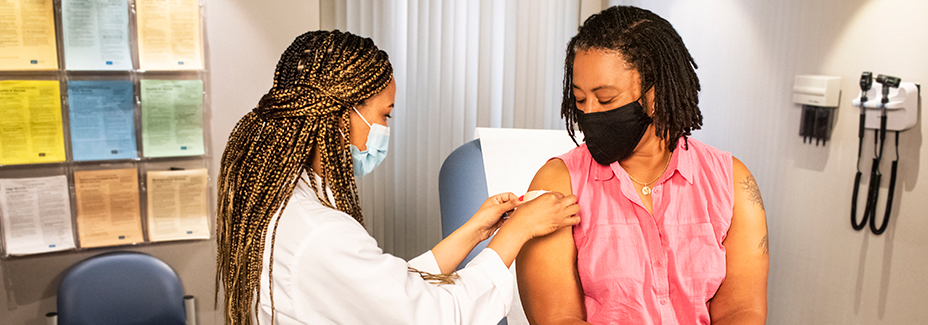
In the U.S., healthcare workers were among the first to be offered a vaccine for COVID-19.
In December, the first vaccines against the deadly coronavirus were distributed in the United States. Many people cheered this scientific breakthrough. It offers hope for an end to a pandemic that has claimed more than 350,000 lives in the U.S. alone.
Two vaccines that fight COVID-19, the contagious disease caused by the coronavirus, have taken center stage. They were created by Pfizer, one of the world’s largest pharmaceutical companies, and Moderna, a biotechnology company based in Cambridge, Massachusetts. Vaccines from other manufacturers are still in development.
The Pfzier and Moderna vaccines have gone through extensive testing and been proven safe and reliable. According to the Food and Drug Administration (FDA), the two vaccines are 94-95% effective at preventing COVID-19 symptoms. Two doses of the vaccine, administered several weeks apart, are needed.
To learn more about the vaccines, I spoke with Peter Szilagyi, a clinical researcher and professor of pediatrics at the University of California, Los Angeles (UCLA). Szilagyi serves as a member of the Advisory Committee on Immunization Practices. This independent group of experts advises the Centers for Disease Control and Prevention (CDC) on all vaccine recommendations in the U.S., including the two new coronavirus vaccines.
In addition to looking for severe side effects when testing vaccines, Szilagyi said, “we look at mild, short-term side effects like pain in the arm, fever, or muscle ache.” Despite causing minor, flu-like symptoms in some patients, the vaccines from Pfizer and Moderna have been demonstrated to be safe for most people.
VACCINES FOR CHILDREN
Unfortunately, distributing the vaccine nationwide will take several months. Already, there are significant delays. According to the CDC, just 2.8 million people in the U.S. have received the first dose of the vaccine to date. The U.S. population exceeds 328 million.
Health care providers and people who live in long-term care facilities have been among the first groups to receive an initial dose of the vaccine. Individuals under the age of 18 will be among the last, in part because very little testing has been done on children.
“If there is going to be a problem with a vaccine, the last thing you want is for the subjects in a vaccine trial to be children,” Szliagyi explained. Also, COVID-19 is typically far more dangerous for adults than it is for children, so it is logical to study the vaccine in adults first.
Szliagyi said that “kids over 12 will most likely be able to get the vaccine by this summer, or before school starts.” For everyone who has experienced learning disruptions during the pandemic, it’s difficult to imagine that the next school year could bring a return to normal.
