KID REPORTERS’ NOTEBOOK
Should Kids Get the COVID-19 Vaccine?
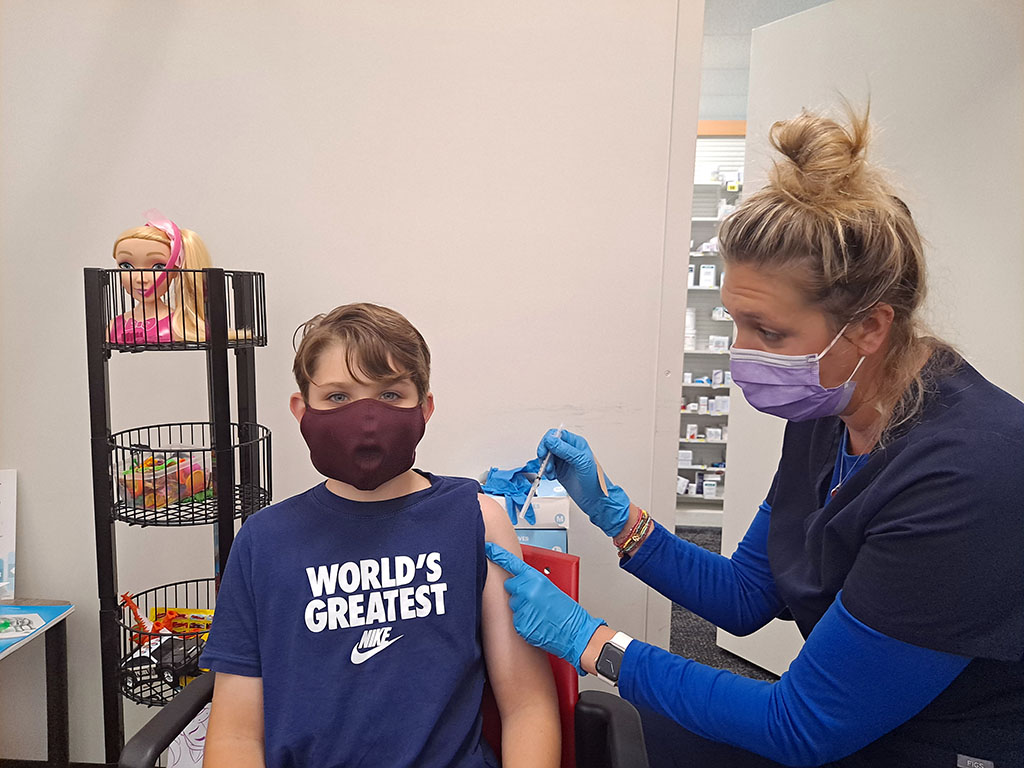
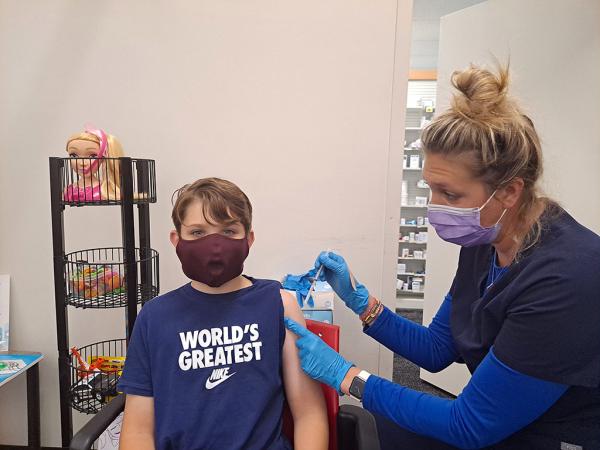
“The shot didn’t hurt,” Benji Miller, 9, said about the coronavirus vaccine.
On October 29, the COVID-19 vaccine for children ages 5-11 was approved by the United States Food and Drug Administration (FDA). The FDA is an agency of the Department of Health and Human Services. It is responsible for protecting public health.
Nearly two years into the coronavirus pandemic, millions of children around the country have been inoculated against COVID-19, the contagious disease caused by the virus. In Parkland, Florida, where I live, nine-year-old Benji Miller was excited to be vaccinated. “The shot didn’t hurt,” Benji said. “I feel more safe now.”
I recently asked Dr. Peter Marks about the vaccine and the approval process. Dr. Marks is the director of the CBER (Center for Biologics Evaluation and Research) at the FDA. He is an expert in cell and molecular biology, hematology, internal medicine, and oncology. Here are his responses, which have been edited for clarity and length.
Can you explain how vaccines are approved?
The people who make the vaccine conduct clinical trials to see that it is safe and that it works. They provide the information about how they make the vaccine, and all of the work they’ve done, to the FDA.
The FDA reviews all of the information about how the vaccine is manufactured and why it’s safe and effective. We actually go to the sites where the work was done. We review information from the clinical trials to make sure that it was collected accurately. Before we give an authorization or approval, we make a determination that the benefits outweigh the risks.
What would you say to families concerned that there was only a small sample size in the vaccine testing process?
For the COVID-19 vaccines, the sample size was not all that small. Usually, when the FDA approves a vaccine, on average, at least 22,000 people have received it. That would be for any vaccine.
The FDA’s job doesn’t stop when we give an authorization or approval. We continue to work with our colleagues at the Centers for Disease Control and Prevention [CDC] and monitor adverse events that occur with a vaccine. We monitor how well the vaccine works in the real world. We have data coming in on millions and millions of people, so even though the clinical trial process was relatively small—tens of thousands of people—the data that we have, that we can rely on, is on millions of people.

Lincoln talks with Dr. Peter Marks of the FDA via video.
Some people argue that most kids don’t have severe symptoms even if they do get COVID-19. Why is it important to be vaccinated?
Even though adults get worse symptoms, some children do get very severe symptoms and end up in the hospital. Unfortunately, a small number of children die from COVID-19. No child, no one, should have to die of a vaccine-preventable illness. That has been our motto, and that's why we vaccinate everyone against measles, mumps, and rubella. It’s why we vaccinate against polio, diphtheria, tetanus, and pertussis [whooping cough]. We don’t want people to get these illnesses. Even just preventing a small number of children from dying is very important.
I’ve heard adults say that they’re not getting vaccinated until they can do their own research, or that the vaccines came out too fast. Why should families trust the FDA?
At the FDA, we’re tasked with making sure that vaccines are high-quality, safe, and that they are effective. These have to be safe and effective for everyone.
We want to be able to use them in our own families and friends, so we take that responsibility very seriously. We stand behind anything that comes out of the FDA, in terms of vaccines, that shows they are safe and effective.
People who are concerned should talk with their health care providers. Although these vaccines were developed relatively rapidly, the process happened with great care, and no corners were cut. All of the things that needed to happen to have a safe vaccine that works, all those check boxes were checked. All of the manufacturing work that needed to happen, all those check boxes were checked. We had to work quickly because so many people were dying from COVID-19.
When you and your colleagues at the CBER realized that the Pfizer vaccine met the criteria for approval, were you emotional?
Yes, we were emotional. There was a tremendous amount of work that went into making this happen in a very timely manner. To have a vaccine that was as effective as these vaccines was quite exciting. Vaccines aren’t always 95% effective. To have a new vaccine that turned out to be that way when we really needed it was quite a gift.
When do you think approval will come for a COVID-19 vaccine for kids under the age of five?
There will probably be an authorization for kids younger than five sometime in 2022, hopefully early in the year. When we get the data, we’ll look it over carefully in a very timely manner, just as we’ve done for the other authorizations and approvals.
Do you think the COVID-19 vaccine will become a yearly requirement like the flu shot?
We don’t know yet how long the immunity will last for the COVID-19 vaccines, how long the disease will continue to circulate, or how much it will change over time.
